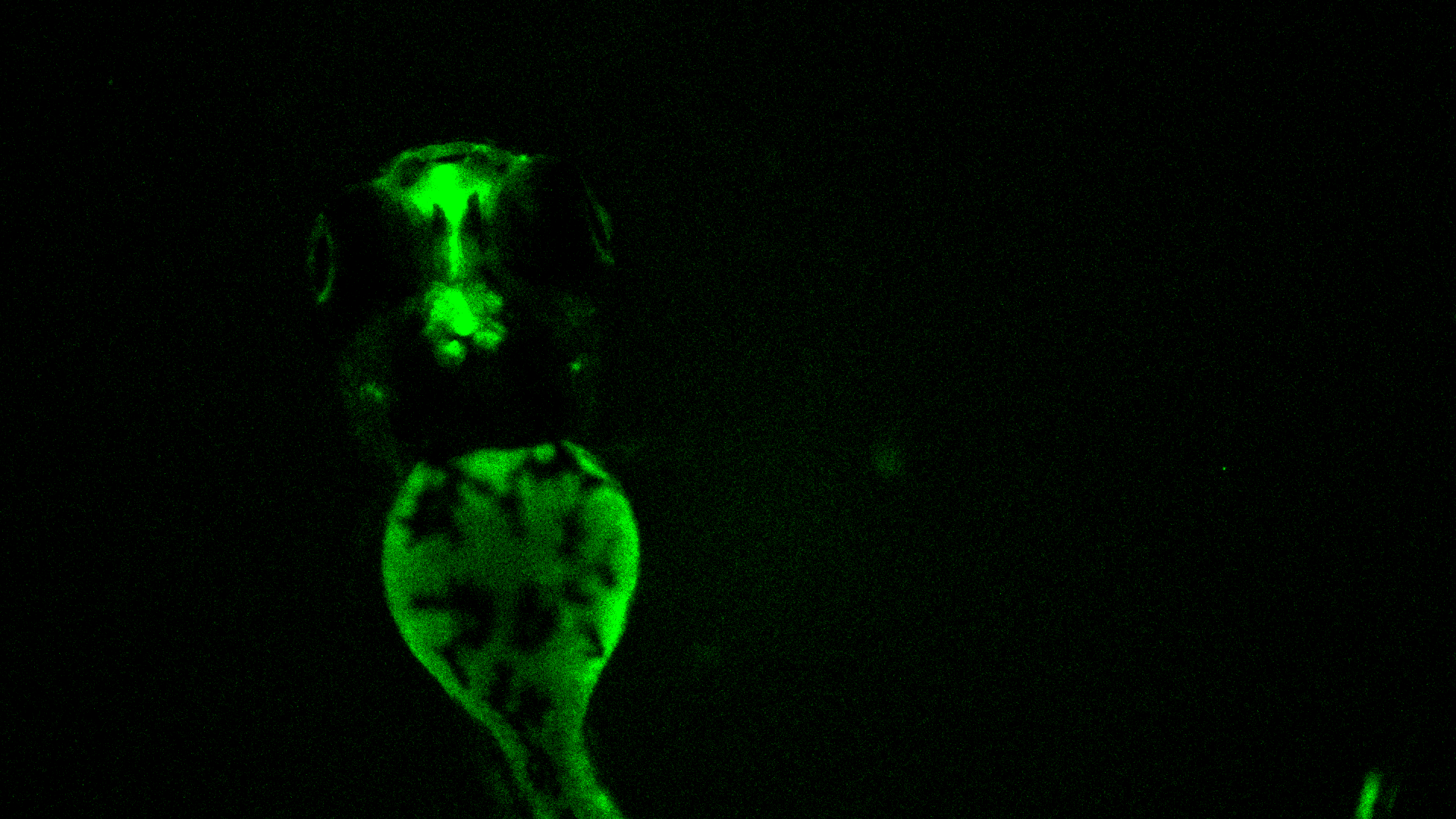
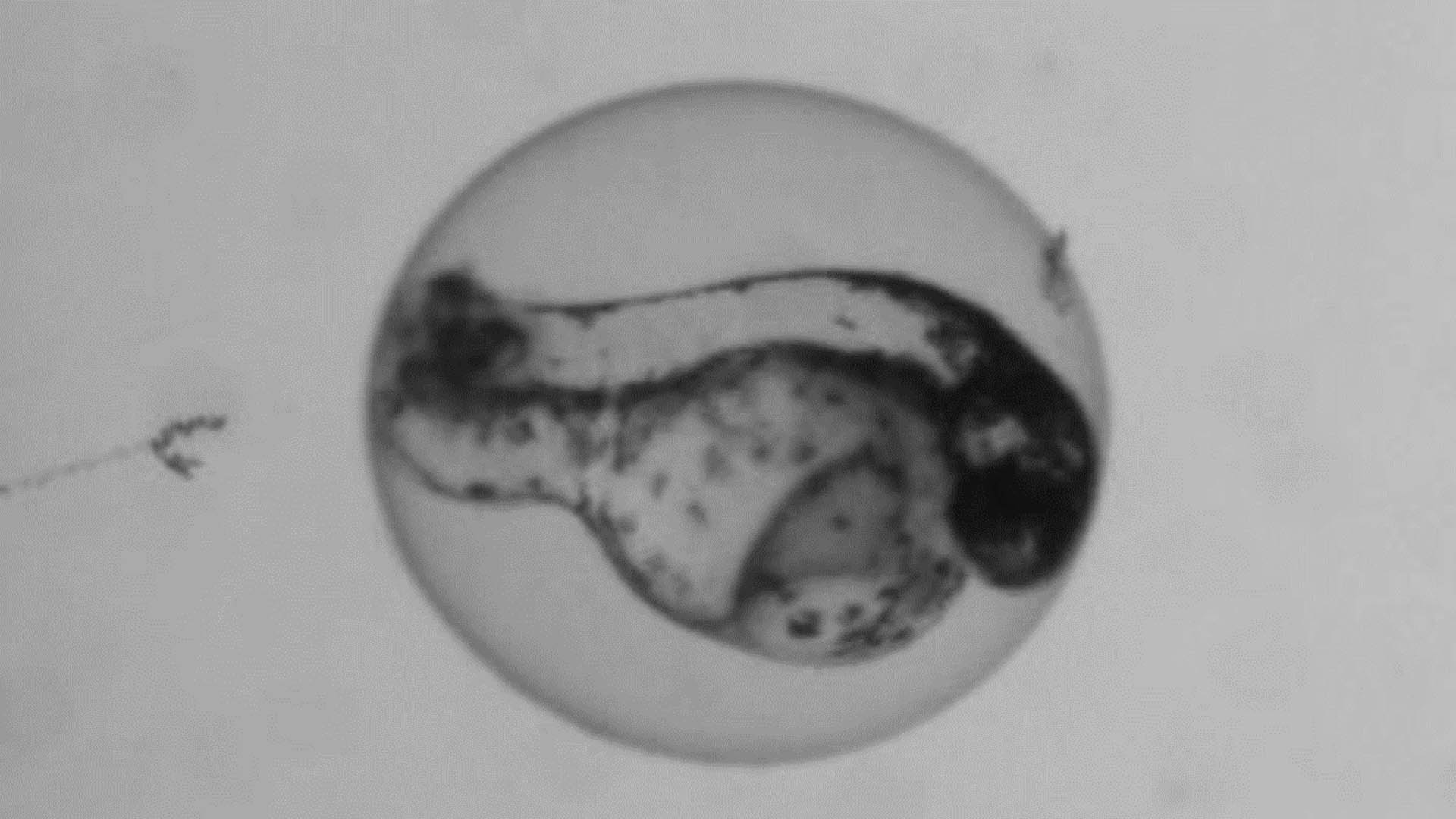
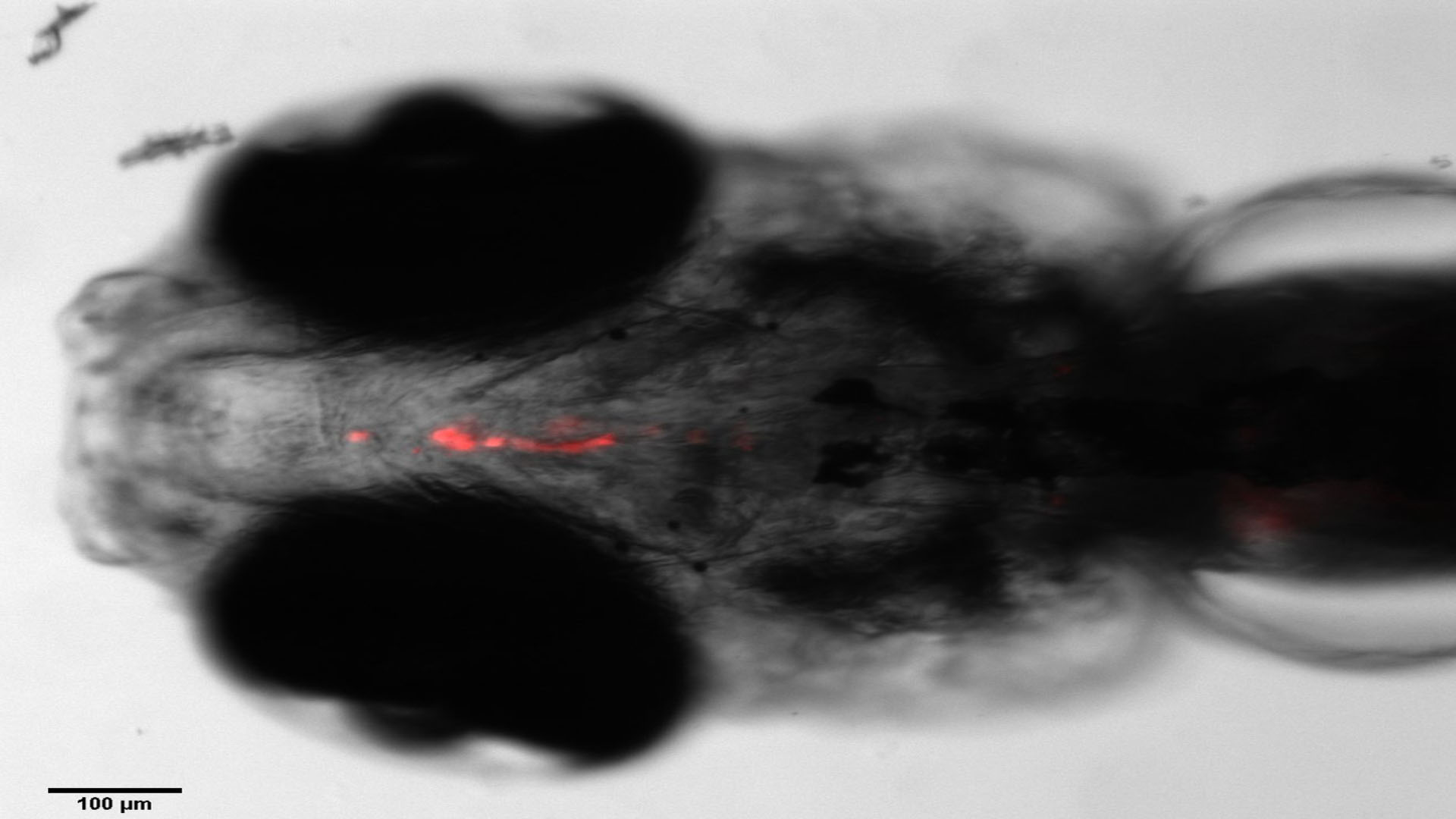
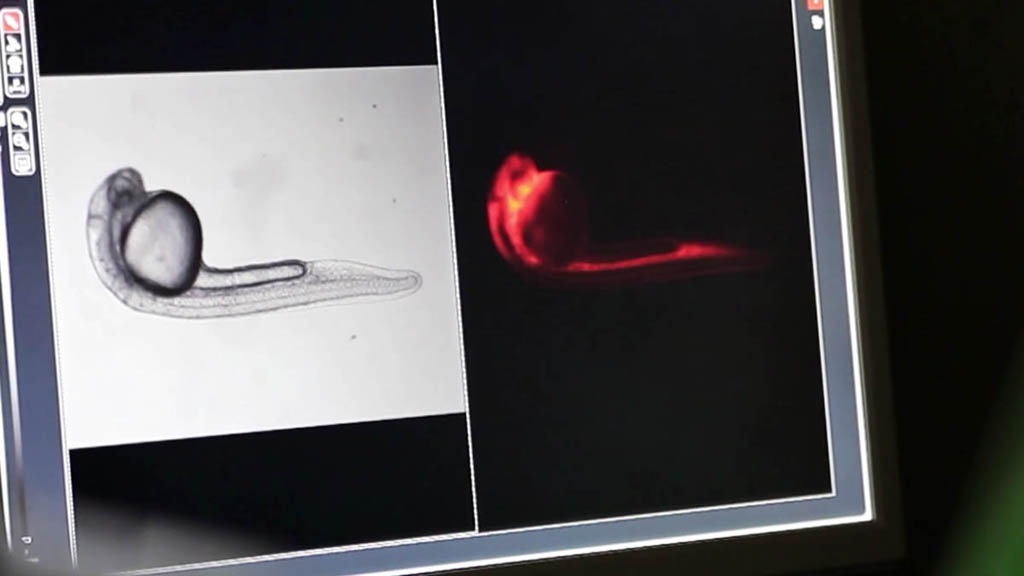
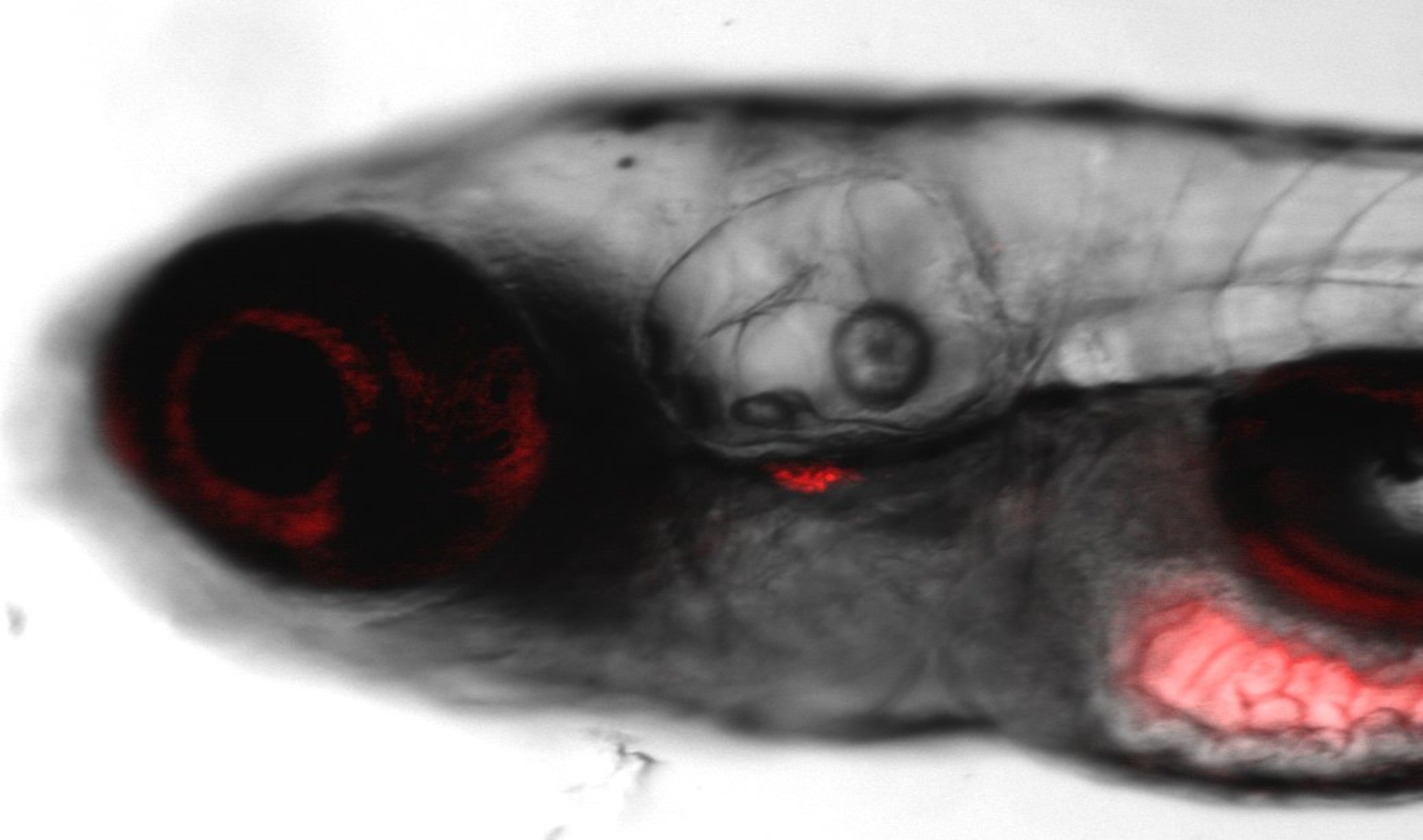
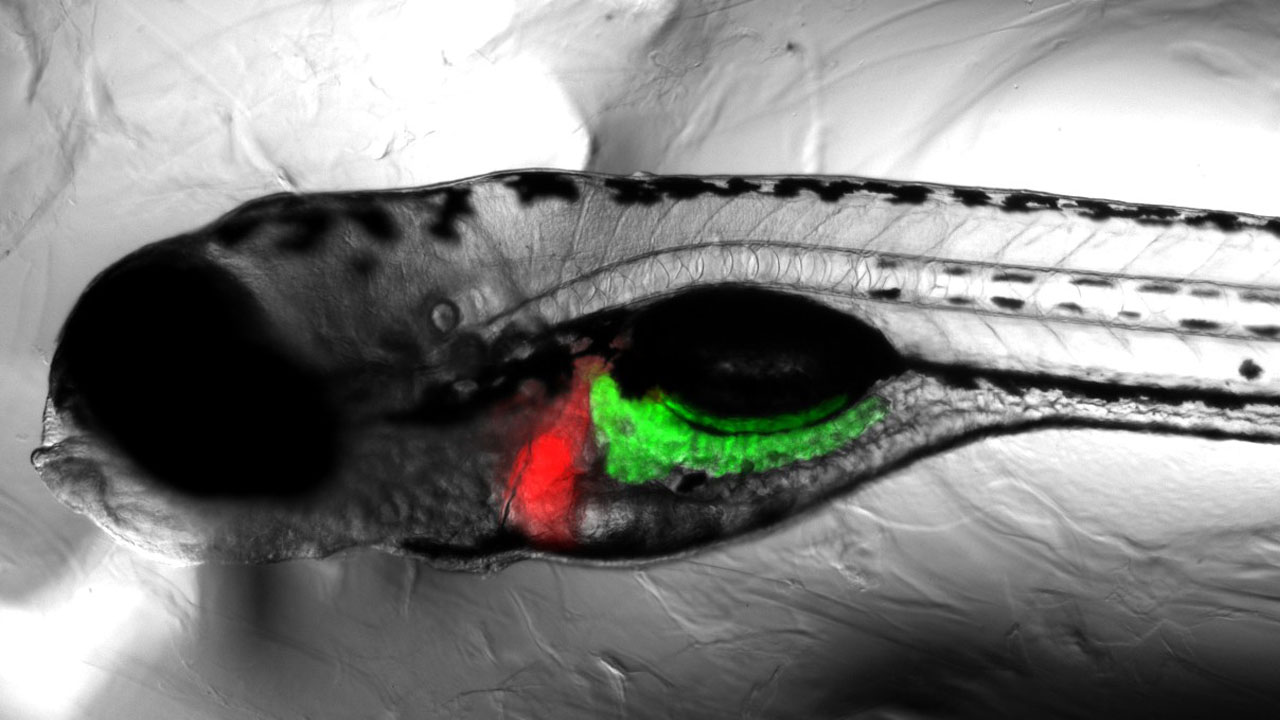
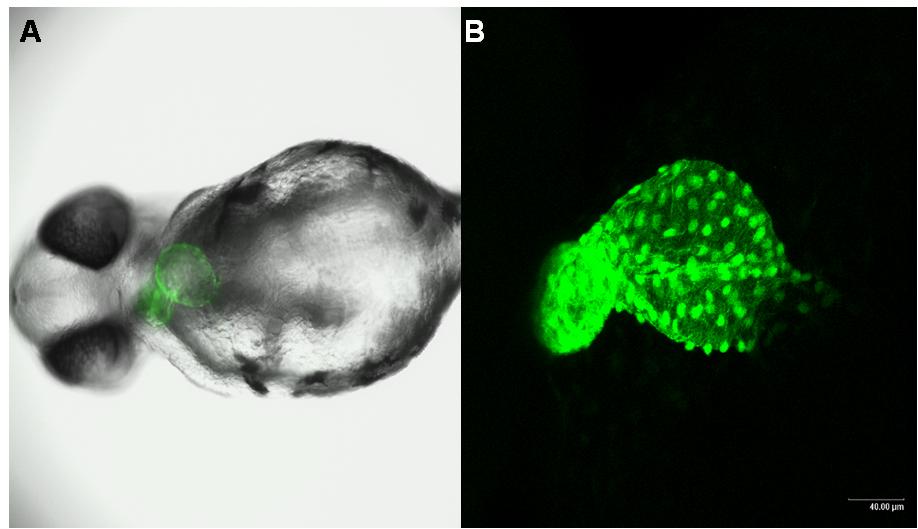
Teratotox Assay
In order to understand the potential of a drug to induce birth defects in offspring, teratogenic studies must be performed. Reproductive toxicity is a major concern and different guidelines, such as the ICH S5(R2) guideline, state the need to assess chemicals safety during the Drug Discovery and Development Process or before drug commercialization.
The zebrafish embryo is an emerging model due to its inherent properties (ease of manipulation, external fertilization and transparency) together with the need to apply the 3Rs. This model has a high genetic homology with humans (over 85%) as well as important parallels in organogenesis and functional mechanisms.
Services
-
EASZY Assay: Estrogen Pathway
Ecotox-Assays / Specific-Toxicity-Assays / -
Zebrafish Sperm Cryopreservation and In Vitro Fertilization
Specific-Toxicity-Assays / -
Zebrafish Models for Amyotrophic Lateral Sclerosis
Specific-Toxicity-Assays / -
Kidney Toxicity
Specific-Toxicity-Assays / -
Sperm quality assessment
Specific-Toxicity-Assays / -
Muscle toxicity
Specific-Toxicity-Assays / -
Acutetox Assay - Ecotox
Ecotox-Assays / -
Daphnia Immobilization
Ecotox-Assays / -
Microplate Alga Growth Inhibition Test
Ecotox-Assays / -
Antioxidation Assay
Efficacy-Assays / -
Regeneration Assay
Efficacy-Assays / -
Melanin Quantification Assay
Efficacy-Assays / -
Thyroid Disruption Assay
Ecotox-Assays / -
Neurodegenerative and Rare Diseases
Efficacy-Assays / Disease-Model-Generation / -
Cancer: Angiogenesis Inhibition Assay
Efficacy-Assays / -
Multitox Assay
Specific-Toxicity-Assays / -
Immunotox Assay
Specific-Toxicity-Assays / -
Ototox Assay
Specific-Toxicity-Assays / -
Neurotox Assay
Specific-Toxicity-Assays / -
Hepatotox Assay
Specific-Toxicity-Assays / -
Cardiotox Assay
Specific-Toxicity-Assays / -
Teratotox Assay
General-Toxicity-Assays / -
Acutetox Assay
General-Toxicity-Assays / -
Disease Model Generation
Disease-Model-Generation / -
Target validation
Target-Validation /