Angiogenesis is a dynamic process by which new blood vessels form from pre-existing vasculature. This process begins with vasculogenesis, the de novo formation of vessels, which then go through further alteration and expansion through angiogenesis. Unlike most other tissues, vasculature exhibits a outstanding regenerative capacity, enabling it to grow and repair damaged tissues even in adult organisms.
Angiogenesis is fundamental to various physiological processes, including embryonic vascular development, tissue differentiation, wound restoration, and organ regeneration. This process is tightly regulated by a balance of pro- and anti-angiogenic factors, and any interference in the genetic expression of these proteins can lead to pathological conditions, including tumor progression, rheumatoid arthritis, and psoriasis.
Cancer, a heterogeneous group of diseases, is characterized by the loss of normal cell regulation mechanisms due to genetic and epigenetic alterations. These changes transform the altered cells into unregulated entities with the capability to proliferate endlessly and invade surrounding tissues, ultimately leading to organ dysfunction and, if untreated, patient mortality. The ability of tumor cells to proliferate and metastasize depend on the induction of angiogenesis to meet the soared demand for oxygen and nutrients, making angiogenesis a critical factor in tumor progression.
The inhibition of angiogenesis represents a promising therapeutic approach for treating angiogenesis-related diseases, including cancer. Angiogenesis inhibitors, such as monoclonal antibodies aiming vascular endothelial growth factor-A (VEGF-A), exemplified by bevacizumab, have shown potential in clinical applications for cancer treatment.
Efficient and cost-effective strategies to study angiogenesis inhibitors are crucial for advancing our understanding of tumor biology and developing groundbreaking therapies. Conventional models, typically mammalian, are time-intensive, costly, and often raise ethical concerns. Consequently, researchers are progressively turning to New Alternative Models (NAMs), such as Zebrafish, which offer unique advantages in preclinical testing. In this review, we will discuss the application of zebrafish models in studying angiogenesis inhibition.
Angiogenesis Inhibition
Angiogenesis is a highly regulated process governed by a delicate balance between pro-angiogenic and anti-angiogenic factors. Under physiological conditions, pro-angiogenic molecules such as vascular endothelial growth factor (VEGF) promote the formation of new blood vessels to meet the metabolic demands of expanding tissues. In contrast, angiogenesis inhibitors disrupt this equilibrium by targeting pro-angiogenic factors, endothelial cells, or their respective receptors.
The primary mechanisms of angiogenesis inhibition include:
- Inhibition of VEGF Signaling: VEGF plays a crucial role in regulating angiogenesis by stimulating endothelial cell proliferation, blood vessel growth, and vascular permeability. Angiogenesis inhibitors can disrupt VEGF signaling by targeting VEGF itself or its receptors, thus preventing the formation of new blood vessels.
- Suppression of Endothelial Cell Proliferation: Some angiogenesis inhibitors impede the proliferation of endothelial cells, which are essential for the formation of the vascular endothelium, thereby hindering the expansion of blood vessels.
- Induction of Endothelial Cell Apoptosis: Certain angiogenesis inhibitors trigger apoptosis (programmed cell death) in endothelial cells, leading to the regression and collapse of established blood vessels.
- Extracellular Matrix Remodeling: Angiogenesis inhibitors may also interfere with the degradation of the extracellular matrix, thereby inhibiting endothelial cell migration and avoiding the construction of new vasculature.
Therapeutic strategies targeting angiogenesis in tumors have led to the development of various classes of drugs, including monoclonal antibodies (e.g., bevacizumab, cetuximab, trastuzumab, panitumumab), tyrosine kinase inhibitors (e.g., axitinib, cabozantinib, pazopanib, regorafenib, sorafenib, sunitinib, vandetanib), and compounds that target the mTOR pathway.
Why Study Angiogenesis Inhibitors Using Zebrafish New Alternative Model (NAM)?
The zebrafish model offers abundant advantages and is recognized by the National Institutes of Health (NIH) as the third most broadly used vertebrate model organism, following mice and rats. Zebrafish embryos are increasingly employed as an in vivo model for studying angiogenesis due to their reliability, cost-effectiveness, and reduced ethical concerns compared to other vertebrate models, in adherence to the 3Rs principle (Replacement, Reduction, and Refinement of animal use). The anatomical structure of the developing vascular tree, the angiogenic process, and the molecular mechanisms underlying vascular formation are strikingly analogous to those observed in humans and higher vertebrates. Moreover, key molecular pathways that regulate angiogenesis in mammals—such as vascular endothelial growth factors (VEGFs), fibroblast growth factors (FGFs), ephrin receptors, and angiopoietins—are conserved in zebrafish.
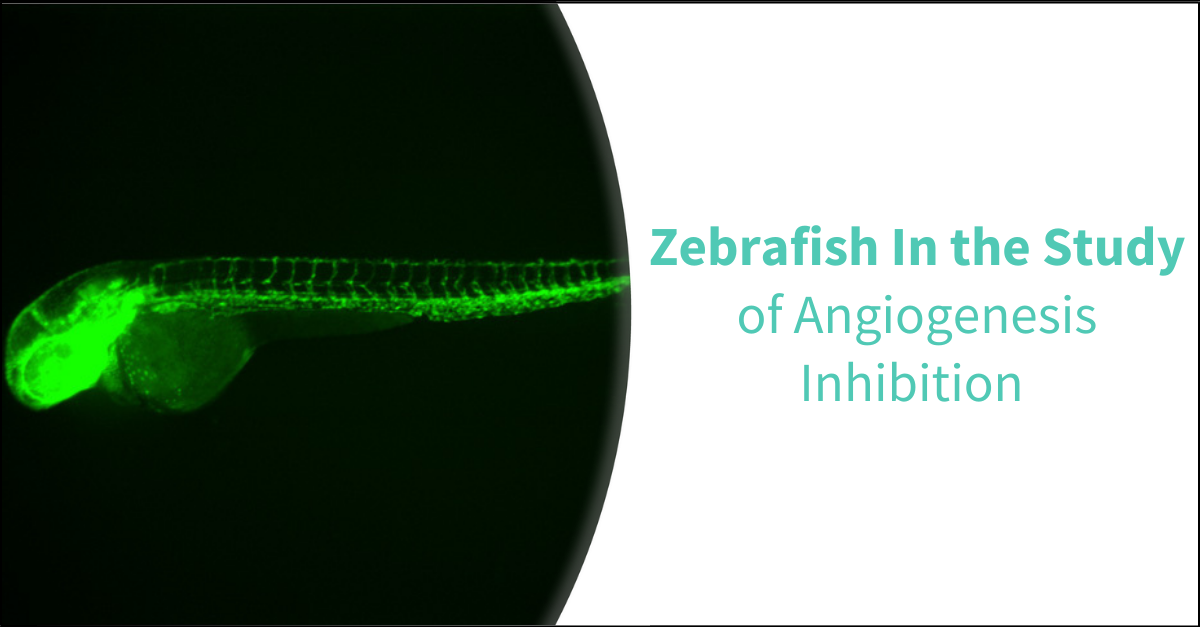
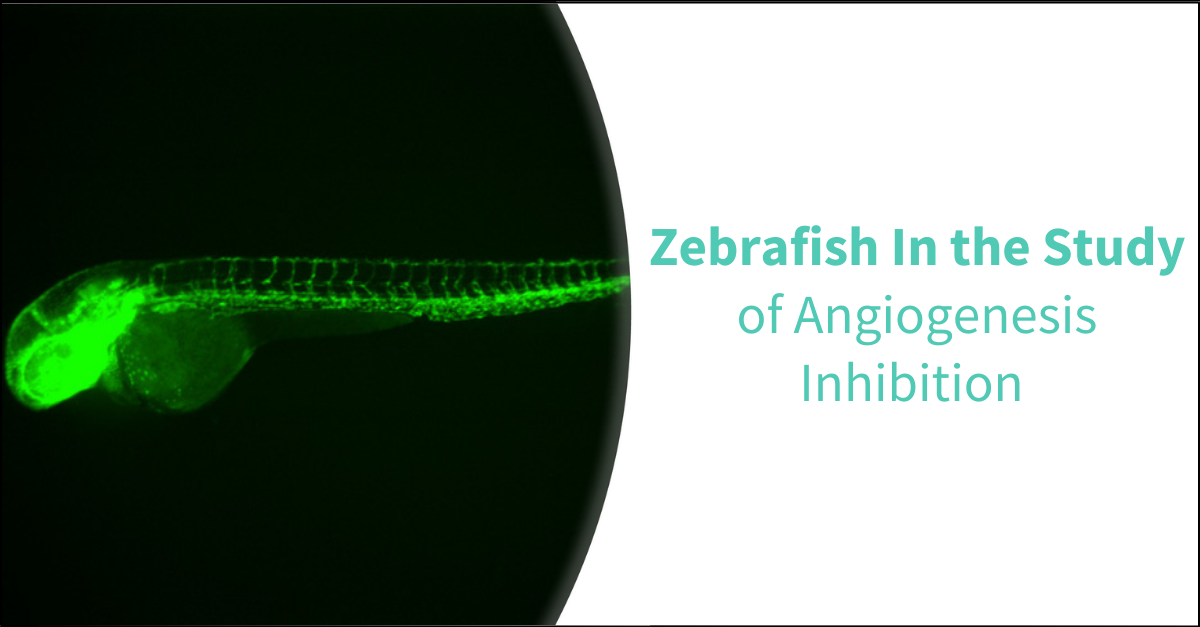
The optical transparency of zebrafish embryos enables direct visualization and real-time tracking of changes within the developing vascular system. The development of fluorescent transgenic reporter lines specific to various vascular components further enhances the study of angiogenesis. Notable lines include the Tg(fli1:egfp), which fluoresces in vascular endothelial cells (ECs), the Tg(lyz:egfp) for myeloid cells (MCs), and the Tg(c-myb:egfp) for hematopoietic stem cells (HSCs).
Additionally, zebrafish embryos are particularly well-suited for angiogenesis studies, as the cardiovascular system is one of the first to develop and function in the embryo. This system includes the heart, blood vessels, and lymphatic vasculature, ensuring efficient circulation and the distribution of oxygen and nutrients throughout the organism.
Angiogenesis assays typically involve treating zebrafish embryos with compounds and assessing phenotypic changes in blood vessel development, such as vessel length, branching patterns, and density. Quantitative analysis of these phenotypic alterations can be performed using advanced fluorescence microscopy or other imaging techniques.
Zebrafish embryos are also ideal for studying regenerative angiogenesis, as vascular injury models such as tail fin amputation or laser-induced wound can be employed. Following injury, angiogenesis inhibitors can be administered to assess their effects on the regenerative and healing processes. Furthermore, gene expression analysis associated to angiogenesis can be conducted employing quantitative PCR to assess the impact of angiogenesis inhibitors on the expression of key angiogenic markers.
Biobide’s Proposal for Angiogenesis Inhibition Assessment
Biobide has established a High Content Screening (HCS) assay meant to assess the ability of compounds to inhibit angiogenesis.
Angiogenic vessels can be easily observed, making them ideal for identifying angiogenesis inhibitors. Utilizing a transgenic zebrafish line with fluorescence specifically in the blood vessels, the intersegmental vessels can be clearly visualized and analyzed. This includes quantification of vessel number and branching patterns. Following a 24-hour compound treatment in zebrafish embryos, fluorescent images are captured, and the fluorescent area, total number of intersegmental vessels, and vessel branching are quantified.
Biobide’s angiogenesis assay has been validated with a scope of reference compounds known for their angiogenesis inhibitory properties, as well as negative control compounds. The assay proves 100% specificity and 83% sensitivity, enabling the early selection of effective candidates during the Drug Discovery and Development process.
Conclusion
Novel therapeutic advances targeting angiogenesis inhibition hold sizable potential for treating countless diseases, particularly cancer. Consequently, efficient preclinical research on new angiogenesis inhibitors is essential.
In this context, Zebrafish has emerged as a valuable preclinical NAM, establishing prompt decision-making during the early stages of the Drug Discovery process. The use of Zebrafish embryos as a model organism for studying angiogenesis inhibitors offers several advantages, supporting a highly reliable and effective platform that adheres to rigorous ethical standards. Additionally, Zebrafish embryos can be employed in High Content Screening (HCS) assays to evaluate the effects of multiple compounds simultaneously, thereby increasing throughput, reducing costs, and minimizing ethical concerns. This enables the efficient identification of potential angiogenesis inhibitors from compound libraries.
Thus, by utilizing Zebrafish models, researchers can gain profound insights into the molecular mechanisms underlying angiogenesis and more accurately assess the efficacy of potential inhibitors, all while enhancing time and cost efficiency.
Sources
- Cancer: Angiogenesis Inhibition Assay | Biobide
- Kunxian Capsule Extract Inhibits Angiogenesis in Zebrafish Embryos via PI3K/AKT-MAPK-VEGF Pathway - PubMed (nih.gov)
- Antiangiogenic potential of Lepista nuda extract suppressing MAPK/p38 signaling-mediated developmental angiogenesis in zebrafish and HUVECs - PubMed (nih.gov)
- Investigation of the Effects of Some Cardiovascular Drugs on Angiogenesis by Transgenic Zebrafish - PMC (nih.gov)
- Di-(2-ethylhexyl) phthalate impairs angiogenesis and hematopoiesis via suppressing VEGF signaling in zebrafish - PubMed (nih.gov)
- Sesamin lacks zebrafish embryotoxicity but exhibits evidence of anti-angiogenesis, anti-oxidant and anti-inflammatory activities - PubMed (nih.gov)
- Angiogenesis in zebrafish - PubMed (nih.gov)
- Imaging Cancer Angiogenesis and Metastasis in a Zebrafish Embryo Model - PubMed (nih.gov)
- Caffeine Inhibits Direct and Indirect Angiogenesis in Zebrafish Embryos - PubMed (nih.gov)